
Curasorb Alloy Development Underway
Terves and Magsorbeo Biomedical continue to make progress on alloy prototyping and testing under our Joint Development Agreement as we develop our Curasorb Alloy technology. A matrix of alloy compositions have been cast and are currently being evaluated. These research-stage chemistries are supporting broader product development of our Akesorb Maxillofacial System and will provide important data points for future expansion into new applications. These experiments will inform us on how to build upon our intellectual property positions and the ability to scale to commercial alloy production under our medical device quality system with Terves. The primary goal of our team is to down-select the most promising alloy prototypes for evaluation in our pilot preclinical study by the end of 2022.
NSF SBIR/STTR Proposal

Our team, led by Carolyn Woldring, successfully submitted a Project Pitch to the National Science Foundation (NSF) in June for a Phase I Grant. Within 24 hours, the NSF accepted our pitch and invited us to submit a full proposal for a Phase I Award under the Small Business Innovation Research (SBIR)/Small Business Technology Transfer (STTR) program. The team is now actively writing the full proposal, along with building the partnerships with facilities that will conduct the pilot preclinical study intended to be funded by this award. Despite having one year from our pitch acceptance date (July 1, 2022) to submit our full proposal, we are on track to submit before the next proposal window closes on October 26, 2022. The NSF states that a final decision regarding the award will be provided within 6 months of submission (April 26, 2023).
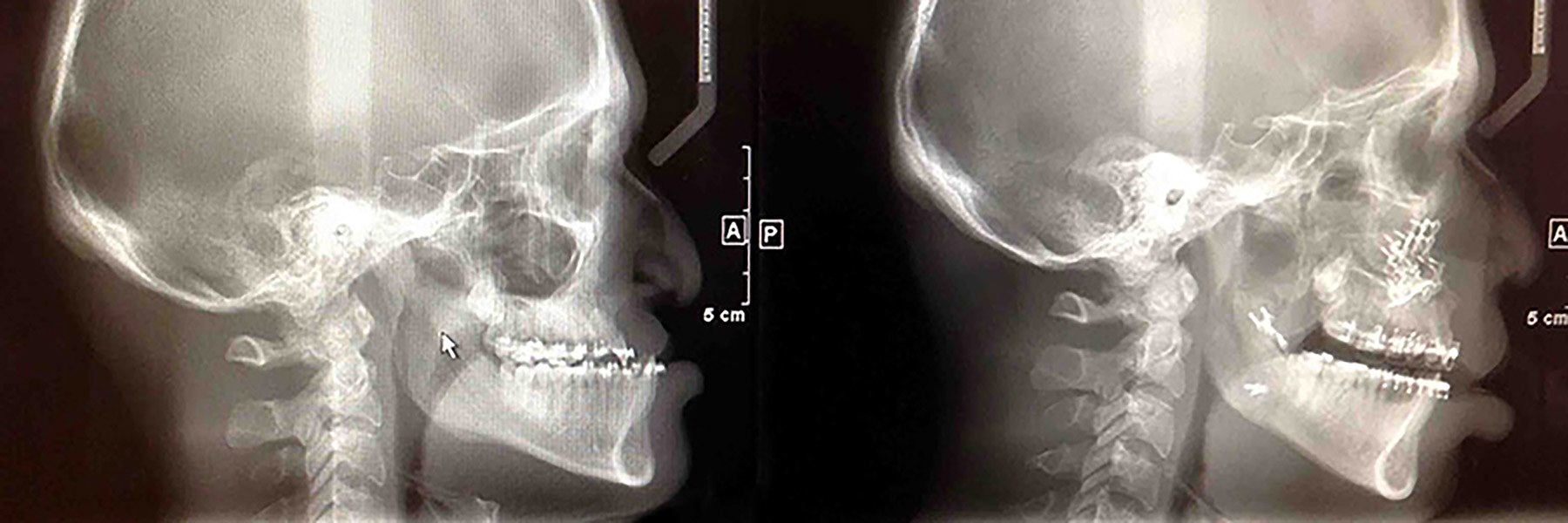
Akesorb System Product Development Launched
Starting in the Development Phase, pending the necessary funding, Magsorbeo will begin supplementing our project team with external contract support. Our most notable partner is Christian Knoepfle, who is a veteran implant designer recognized as a leader among both engineers and surgeons. His experience includes collaborative design with Key Opinion Leaders and commercial launch of both titanium and resorbable polymer systems for craniomaxillofacial fixation.
Magsorbeo Biomedical officially launched its Akesorb Maxillofacial System Product Development Project (PDP) in Q2 2022, focused on commercialization of their Maxillofacial Fixation System. We have closed on the Concept Phase and are well into the Feasibility Phase of the project. A thorough literature review has been conducted and is currently being summarized for the report. Design inputs for the fixation system, including user requirements and hazards have been drafted. As applying magnesium to fixation systems has been well-proven, the Feasibility Phase will concentrate on the feasibility of the Curasorb Alloy technology, characterizing its absorption profile and mechanical properties, optimizing process settings, and designing the alloy for future commercialization. We will launch the Development Phase in August to focus on tuning Curasorb properties to best meet the needs of the Akesorb System, starting with mechanical design and testing of the screws and plates to prepare for our pilot preclinical study.
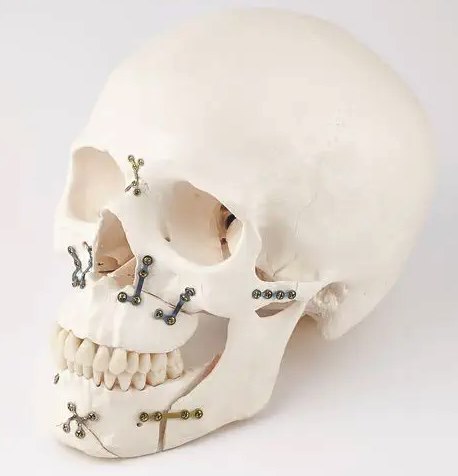
Quality Assurance and Regulatory Affairs
Over the past several months Leann Christman has been working with our team to build our ISO 13485 and 21CFR 820 Quality Management System. This system is currently being utilized in all product development activities and will be expanded into the Terves manufacturing facility during pre-production and commercialization. To support the operation of our quality system, Magsorbeo Biomedical is happy to welcome Madelyn Christman as our QARA Coordinator.
In parallel with our quality system and product development activities, we have defined our Regulatory Strategy and Plan for our Akesorb System. Our first interaction with FDA will come later this year through our first FDA Pre-submission. Through this interaction, we expect to feedback from FDA regarding the regulatory plan and validation test plans. Magsorbeo will use this feedback to finalize Regulatory Plan and Testing Validation Plans that will confirm development timelines and budgets. The main objective to the Pre- submissions is to reduce project risk.



